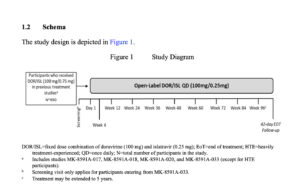
A Phase 3 Open-label Clinical Study of Doravirine/Islatravir (DOR/ISL [100 mg/0.25 mg]) Once Daily for the Treatment of HIV-1 Infection in Participants Who Previously Received DOR/ISL (100 mg/0.75 mg) QD in a Phase 3 Clinical Study
Hypotheses, Objectives, and Endpoints:
Hypotheses are aligned with objectives in the Objectives and Endpoints table.
There are no hypotheses to be tested in this study. The following objectives will be evaluated in adults with HIV-1 who received DOR/ISL in previous DOR/ISL treatment studies:
| Primary Objective | Primary Endpoint |
|---|---|
| To evaluate the safety and tolerability of DOR/ISL (100 mg/0.25 mg) as assessed by accumulated safety data through Week 96 | Adverse events Adverse events leading to discontinuation of DOR/ISL |
| Secondary Objectives | Secondary Endpoints |
To evaluate the antiretroviral activity of DOR/ISL (100 mg/0.25 mg) as assessed by the percentage of participants with the following at Week 96:
|
HIV-1 RNA |
| To evaluate the development of viral resistance to DOR/ISL (100 mg/0.25 mg) | Viral resistance-associated substitutions |
| Study Phase | Phase 3 |
| Primary Purpose | Treatment |
| Indication | HIV infection |
| Population | Adult participants with HIV-1 who received DOR/ISL (100 mg/0.75 mg tablet) in previous MSD-sponsored treatment studies (MK-8591A-017, -018, -020, and -033 [except for HTE participants]) and agree to continue open-label treatment with DOR/ISL until it becomes commercially accessible. |
| Study Type | Interventional |
| Intervention Model | Single Group This is a multi site study. |
| Type of Control | No Treatment Control |
| Study Blinding | Unblinded open-label |
| Blinding Roles | No blinding |
| Estimated Duration of Study | The Sponsor estimates that the study will require approximately 3 years from the time the first participant (or their legally acceptable representative) provides documented informed consent until the last participant’s last study-related contact. |
Number of Participants:
Approximately 650 participants will be enrolled upon completion of studies of DOR/ISL (100 mg/0.75 mg) QD. Enrollment will be adjusted as necessary, based on enrollment in DOR/ISL treatment studies.
| Arm Name | Intervention Name | Unit Dose Strength(s) | Dosage Level(s) | Route of Administration | Regimen/ Treatment Period/ Vaccination Regimen | Use |
|---|---|---|---|---|---|---|
| MK 8591A | doravirine/ islatravir | 100 mg doravirine/ 0.25 mg islatravir | 100 mg doravirine/ 0.25 mg islatravir QD | Oral | Day 1 to Week 96 | Test Product |
Admin.=administration; QD=once daily
Note: Study intervention will be dispensed at the last regularly scheduled study visit for participants who become pregnant. The visit schedule for these participants will be extended through the duration of the pregnancy. Study intervention will be dispensed every 12 weeks during pregnancy, as applicable.
| Total Number of Intervention Groups/Arms | 1 |
| Duration of Participation | Each participant will participate in the study for approximately 102 weeks from the time the participant provides documented informed consent through the final contact. After confirmation of eligibility, each participant will receive study intervention for approximately 96 weeks. After the end of treatment, all participants will be followed for 6 weeks (42 days). Participants who discontinue study intervention or who become pregnant will be followed as described in the protocol. |
| Executive Oversight Committee | No |
| Data Monitoring Committee | No |
| Clinical Adjudication Committee | No |
Study governance considerations are outlined in Appendix 1.
Study Accepts Healthy Participants: No
A list of abbreviations is in Appendix 11.